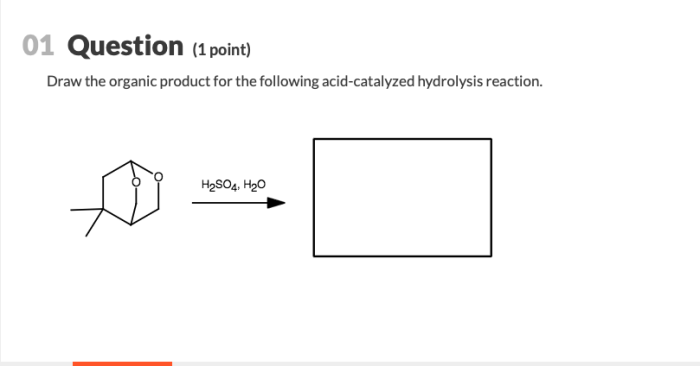
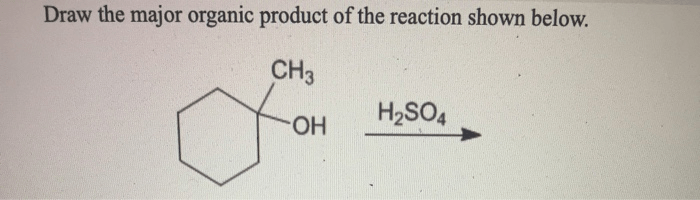
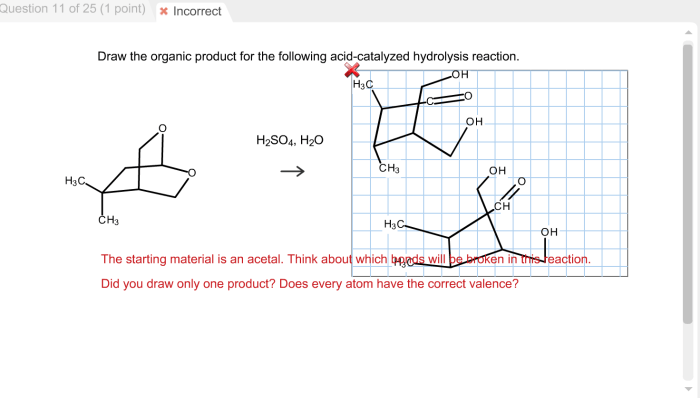
Draw the organic product for the following acid-catalyzed hydrolysis reaction – In the realm of organic chemistry, understanding the mechanisms and applications of acid-catalyzed hydrolysis reactions is of paramount importance. This comprehensive guide delves into the intricacies of these reactions, providing a detailed roadmap for identifying the organic product and exploring their versatile applications.
Acid-catalyzed hydrolysis reactions are widely employed in the synthesis of a vast array of organic compounds, including pharmaceuticals, polymers, and fine chemicals. By mastering the principles governing these reactions, chemists can harness their power for the targeted synthesis of complex molecules.
Organic Product Identification: Draw The Organic Product For The Following Acid-catalyzed Hydrolysis Reaction
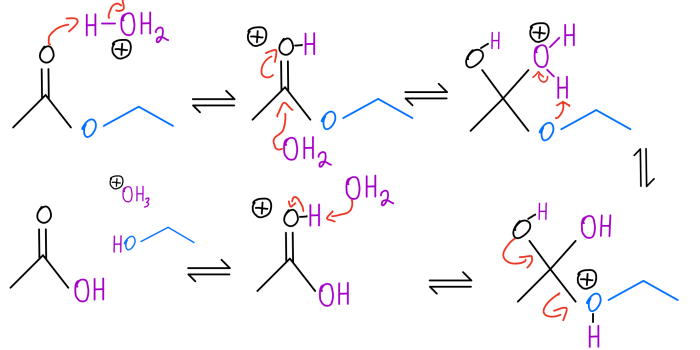
In acid-catalyzed hydrolysis reactions, an ester or amide reacts with water in the presence of an acid catalyst to form a carboxylic acid and an alcohol or amine, respectively.
The mechanism involves a nucleophilic attack by water on the carbonyl carbon of the ester or amide, followed by protonation of the resulting tetrahedral intermediate. The protonated intermediate then collapses to form the carboxylic acid and alcohol or amine products.
The role of the acid catalyst is to protonate the water molecule, making it a more effective nucleophile. The acid also helps to stabilize the tetrahedral intermediate by resonance.
Reaction Conditions
The optimal reaction conditions for acid-catalyzed hydrolysis depend on the specific ester or amide being used. However, in general, the reaction is carried out in an aqueous solution at a temperature between 25 and 100 °C. The acid catalyst can be either a strong acid, such as hydrochloric acid or sulfuric acid, or a weak acid, such as acetic acid.
Reaction Mechanism
The nucleophilic attack mechanism involved in acid-catalyzed hydrolysis can be illustrated as follows:
RCOOR’ + H2O → RCOOH + R’OH
The first step is the nucleophilic attack by water on the carbonyl carbon of the ester or amide. This forms a tetrahedral intermediate.
RCOOR’ + H2O → [RCO(OH)OR’] –
The tetrahedral intermediate is then protonated by the acid catalyst.
[RCO(OH)OR’]–+ H +→ [RCO(OH 2)OR’] +
The protonated intermediate then collapses to form the carboxylic acid and alcohol or amine products.
[RCO(OH2)OR’] +→ RCOOH + R’OH
Regioselectivity and Stereoselectivity
Acid-catalyzed hydrolysis reactions can be either regiospecific or stereospecific. Regioselectivity refers to the preference for one regioisomer over another, while stereoselectivity refers to the preference for one stereoisomer over another.
The regioselectivity of acid-catalyzed hydrolysis reactions is determined by the stability of the tetrahedral intermediate. The more stable the intermediate, the more likely it is to form. The stereoselectivity of acid-catalyzed hydrolysis reactions is determined by the transition state leading to the tetrahedral intermediate.
The transition state with the lowest energy will lead to the formation of the more stable stereoisomer.
Applications of Acid-Catalyzed Hydrolysis, Draw the organic product for the following acid-catalyzed hydrolysis reaction
Acid-catalyzed hydrolysis is a versatile reaction that has a wide range of applications in organic synthesis. Some of the most common applications include:
- The synthesis of carboxylic acids
- The synthesis of alcohols
- The synthesis of amines
- The hydrolysis of proteins
- The hydrolysis of polysaccharides
FAQ Overview
What is the role of the acid catalyst in acid-catalyzed hydrolysis reactions?
The acid catalyst protonates the carbonyl group of the ester, making it more susceptible to nucleophilic attack by water.
What are the factors that influence regioselectivity and stereoselectivity in acid-catalyzed hydrolysis reactions?
Regioselectivity is influenced by the steric and electronic effects of the substituents on the ester, while stereoselectivity is influenced by the conformation of the ester and the approach of the nucleophile.
What are some common applications of acid-catalyzed hydrolysis reactions?
Acid-catalyzed hydrolysis reactions are used in the synthesis of pharmaceuticals, polymers, and other organic compounds. They are also used in the hydrolysis of fats and oils.